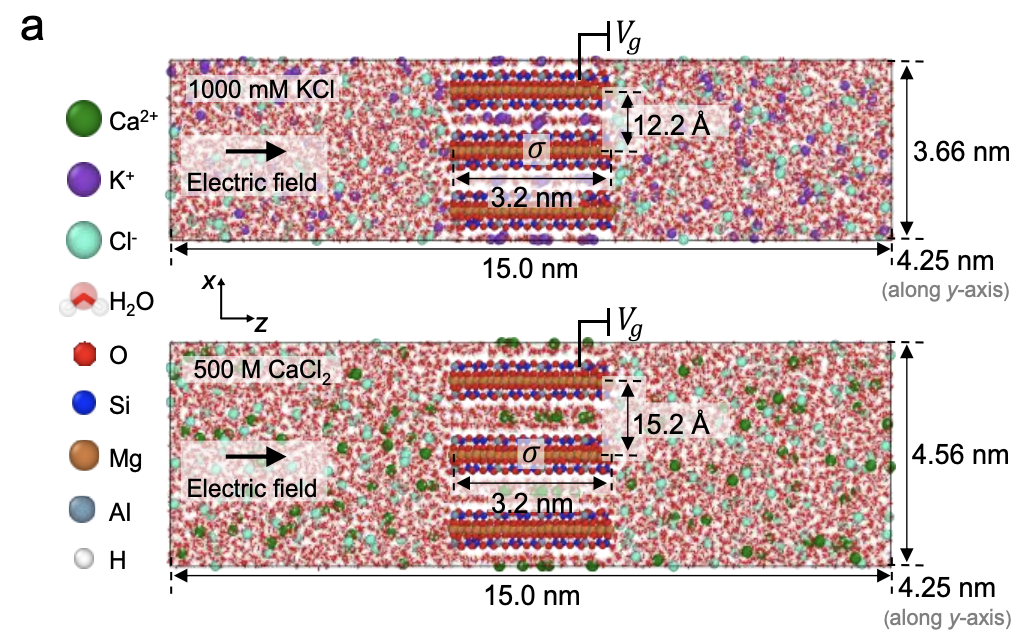
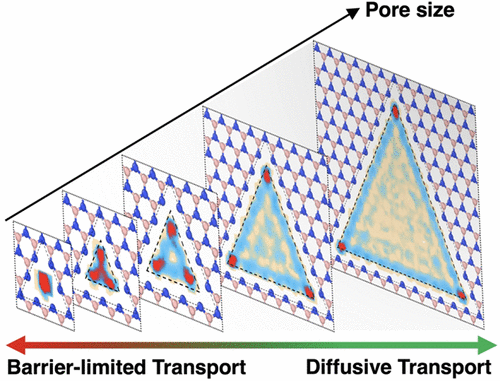
Our comprehensive molecular dynamics research on the transition between diffusion- to barrier-limited transport is published in the Journal of Physical Chemisty B.
Our comprehensive molecular dynamics research on the transition between diffusion- to barrier-limited transport is published in the Journal of Physical Chemisty B.
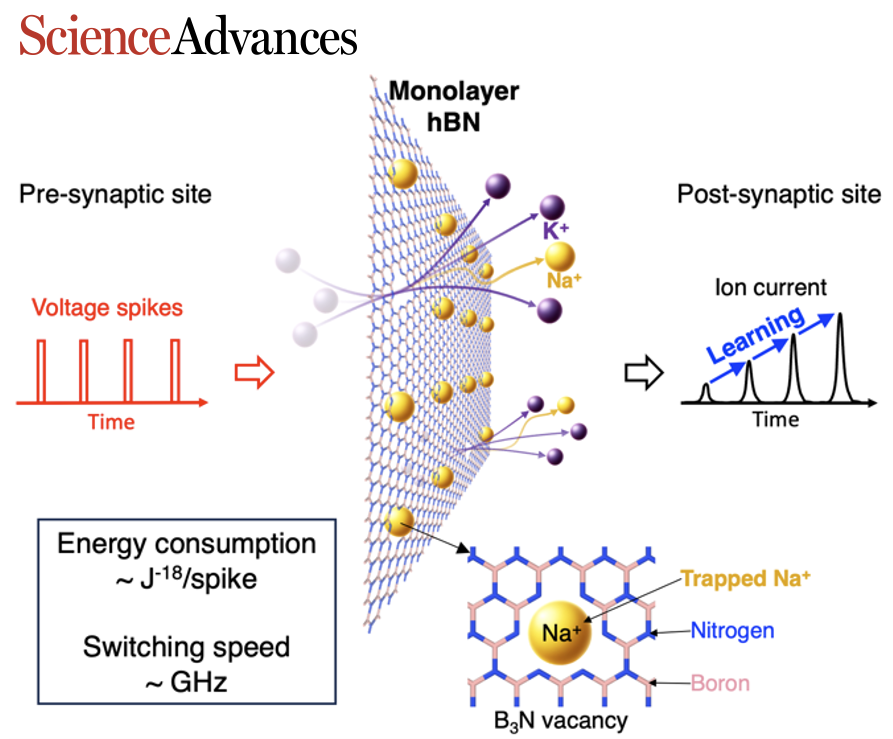
Subdiffusive transport of aqueous ions is an interfacial phenomenon where transport is governed by local free energy barriers. It underlies fascinating phenomenology, including highly selective transport, mechanosensitive phenomena, as well as memristive effects. Although leveraging subdiffusive transport in nanofluidic applications is a relatively recent idea within the nanofluidic community, biological organisms have long exploited it for sophisticated functions such as neurotransmission, sensory perception, and molecular filtration. Despite its significance, our understanding of the transition from diffusive to subdiffusive transport remains rudimentary. Existing literature in fact often postulates that the transition occurs as the pore size decreases below nanometer scale on the broad basis of “steric confinement.”
Visit Link for the full manuscript.